ipyrad command line tutorial - Part I
This is the first part of the full tutorial for the command line interface (CLI) for ipyrad. In this tutorial we’ll walk through the entire assembly and analysis process. This is meant as a broad introduction to familiarize users with the general workflow, and some of the parameters and terminology. We will use simulated paired-end ddRAD data as an example in this tutorial, however, you can follow along with one of the other example datasets if you like and although your results will vary the procedure will be identical.
If you are new to RADseq analyses, this tutorial will provide a simple overview of how to execute ipyrad, what the data files look like, how to check that your analysis is working, and what the final output formats will be. We will also cover how to run ipyrad on a cluster and how to do so efficiently.
Each grey cell in this tutorial indicates a command line interaction.
Lines starting with $ indicate a command that should be executed
in a terminal connected to the Habanero cluster, for example by copying and
pasting the text into your terminal. Elements in code cells surrounded
by angle brackets (e.g.
## Example Code Cell.
## Create an empty file in my home directory called `watdo.txt`
$ touch ~/watdo.txt
## Print "wat" to the screen
$ echo "wat"
wat
Overview of Assembly Steps
Very roughly speaking, ipyrad exists to transform raw data coming off the sequencing instrument into output files that you can use for downstream analysis.
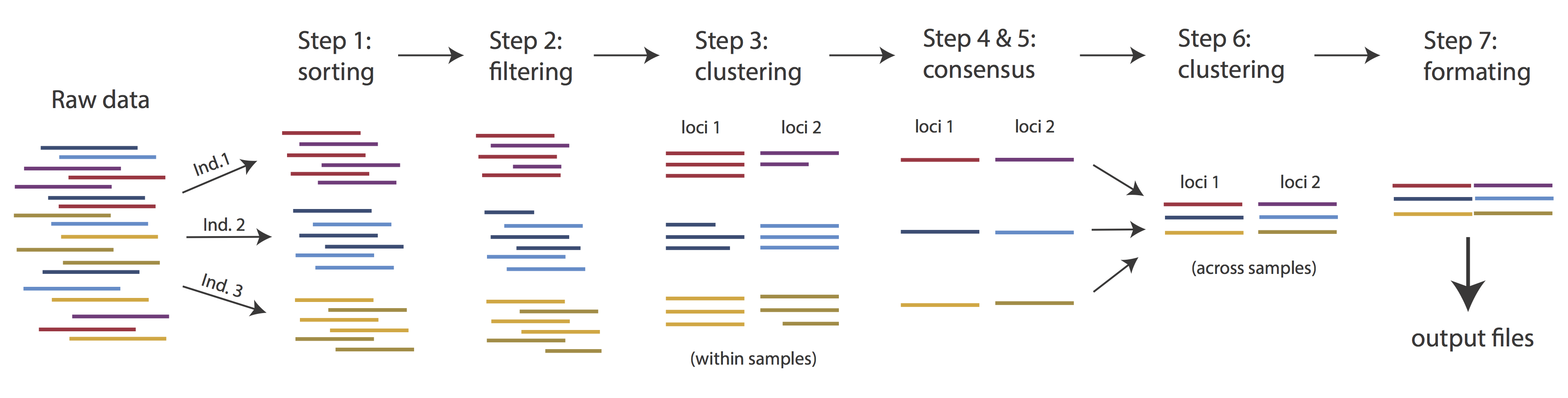
The basic steps of this process are as follows:
- Step 1 - Demultiplex/Load Raw Data
- Step 2 - Trim and Quality Control
- Step 3 - Cluster or reference-map within Samples
- Step 4 - Calculate Error Rate and Heterozygosity
- Step 5 - Call consensus sequences/alleles
- Step 6 - Cluster across Samples
- Step 7 - Apply filters and write output formats
Note on files in the project directory: Assembling RADseq type sequence data requires a lot of different steps, and these steps generate a lot of intermediary files. ipyrad organizes these files into directories, and it prepends the name of your assembly to each directory with data that belongs to it. One result of this is that you can have multiple assemblies of the same raw data with different parameter settings and you don’t have to manage all the files yourself! (See Branching assemblies for more info). Another result is that you should not rename or move any of the directories inside your project directory, unless you know what you’re doing or you don’t mind if your assembly breaks.
Getting Started
We will be running through the assembly of simulated data on a binder instance, so if you haven’t already, please launch the ipyrad repo, and open a New>Terminal.
ipyrad help
To better understand how to use ipyrad, let’s take a look at the help argument. We will use some of the ipyrad arguments in this tutorial (for example: -n, -p, -s, -c, -r). But, the complete list of optional arguments and their explanation is below.
$ ipyrad -h
usage: ipyrad [-h] [-v] [-r] [-f] [-q] [-d] [-n NEW] [-p PARAMS] [-s STEPS] [-b [BRANCH [BRANCH ...]]]
[-m [MERGE [MERGE ...]]] [-c cores] [-t threading] [--MPI] [--ipcluster [IPCLUSTER]]
[--download [DOWNLOAD [DOWNLOAD ...]]]
optional arguments:
-h, --help show this help message and exit
-v, --version show program's version number and exit
-r, --results show results summary for Assembly in params.txt and exit
-f, --force force overwrite of existing data
-q, --quiet do not print to stderror or stdout.
-d, --debug print lots more info to ipyrad_log.txt.
-n NEW create new file 'params-{new}.txt' in current directory
-p PARAMS path to params file for Assembly: params-{assembly_name}.txt
-s STEPS Set of assembly steps to run, e.g., -s 123
-b [BRANCH [BRANCH ...]]
create new branch of Assembly as params-{branch}.txt, and can be used to drop samples from
Assembly.
-m [MERGE [MERGE ...]]
merge multiple Assemblies into one joint Assembly, and can be used to merge Samples into one
Sample.
-c cores number of CPU cores to use (Default=0=All)
-t threading tune threading of multi-threaded binaries (Default=2)
--MPI connect to parallel CPUs across multiple nodes
--ipcluster [IPCLUSTER]
connect to running ipcluster, enter profile name or profile='default'
--download [DOWNLOAD [DOWNLOAD ...]]
download fastq files by accession (e.g., SRP or SRR)
* Example command-line usage:
ipyrad -n data ## create new file called params-data.txt
ipyrad -p params-data.txt -s 123 ## run only steps 1-3 of assembly.
ipyrad -p params-data.txt -s 3 -f ## run step 3, overwrite existing data.
* HPC parallelization across 32 cores
ipyrad -p params-data.txt -s 3 -c 32 --MPI
* Print results summary
ipyrad -p params-data.txt -r
* Branch/Merging Assemblies
ipyrad -p params-data.txt -b newdata
ipyrad -m newdata params-1.txt params-2.txt [params-3.txt, ...]
* Subsample taxa during branching
ipyrad -p params-data.txt -b newdata taxaKeepList.txt
* Download sequence data from SRA into directory 'sra-fastqs/'
ipyrad --download SRP021469 sra-fastqs/
* Documentation: http://ipyrad.readthedocs.io
Create a new parameters file
ipyrad uses a text file to hold all the parameters for a given assembly.
Start by creating a new parameters file with the -n flag. This flag
requires you to pass in a name for your assembly. In the example we use
peddrad but the name can be anything at all. Once you start
analysing your own data you might call your parameters file something
more informative, like the name of your organism and some details on the
settings.
# First, make sure you're in your workshop directory
$ cd ~/ipyrad-workshop
# Now create a new params file named 'peddrad'
$ ipyrad -n peddrad
This will create a file in the current directory called params-peddrad.txt.
The params file lists on each line one parameter followed by a ## mark,
then the name of the parameter, and then a short description of its purpose.
Lets take a look at it.
$ cat params-peddrad.txt
------- ipyrad params file (v.0.9.13)-------------------------------------------
peddrad ## [0] [assembly_name]: Assembly name. Used to name output directories for assembly steps
/home/jovyan/ipyrad-workshop ## [1] [project_dir]: Project dir (made in curdir if not present)
## [2] [raw_fastq_path]: Location of raw non-demultiplexed fastq files
## [3] [barcodes_path]: Location of barcodes file
## [4] [sorted_fastq_path]: Location of demultiplexed/sorted fastq files
denovo ## [5] [assembly_method]: Assembly method (denovo, reference)
## [6] [reference_sequence]: Location of reference sequence file
rad ## [7] [datatype]: Datatype (see docs): rad, gbs, ddrad, etc.
TGCAG, ## [8] [restriction_overhang]: Restriction overhang (cut1,) or (cut1, cut2)
5 ## [9] [max_low_qual_bases]: Max low quality base calls (Q<20) in a read
33 ## [10] [phred_Qscore_offset]: phred Q score offset (33 is default and very standard)
6 ## [11] [mindepth_statistical]: Min depth for statistical base calling
6 ## [12] [mindepth_majrule]: Min depth for majority-rule base calling
10000 ## [13] [maxdepth]: Max cluster depth within samples
0.85 ## [14] [clust_threshold]: Clustering threshold for de novo assembly
0 ## [15] [max_barcode_mismatch]: Max number of allowable mismatches in barcodes
0 ## [16] [filter_adapters]: Filter for adapters/primers (1 or 2=stricter)
35 ## [17] [filter_min_trim_len]: Min length of reads after adapter trim
2 ## [18] [max_alleles_consens]: Max alleles per site in consensus sequences
0.05 ## [19] [max_Ns_consens]: Max N's (uncalled bases) in consensus (R1, R2)
0.05 ## [20] [max_Hs_consens]: Max Hs (heterozygotes) in consensus (R1, R2)
4 ## [21] [min_samples_locus]: Min # samples per locus for output
0.2 ## [22] [max_SNPs_locus]: Max # SNPs per locus (R1, R2)
8 ## [23] [max_Indels_locus]: Max # of indels per locus (R1, R2)
0.5 ## [24] [max_shared_Hs_locus]: Max # heterozygous sites per locus
0, 0, 0, 0 ## [25] [trim_reads]: Trim raw read edges (R1>, <R1, R2>, <R2) (see docs)
0, 0, 0, 0 ## [26] [trim_loci]: Trim locus edges (see docs) (R1>, <R1, R2>, <R2)
p, s, l ## [27] [output_formats]: Output formats (see docs)
## [28] [pop_assign_file]: Path to population assignment file
## [29] [reference_as_filter]: Reads mapped to this reference are removed in step 3
In general the defaults are sensible, and we won’t mess with them for now, but there are a few parameters we must change: the path to the raw data and the barcodes file, the dataype, and the restriction overhang sequence(s).
Fortunately jupyter includes a simple text editor, so select the tab
with the jupyter dashboard, and navigate to the ipyrad-workshop directory.
You can now click on the params-peddrad.txt and it will open in the editor
in a new tab.
We need to specify where the raw data files are located, the type of data we are using (.e.g., ‘gbs’, ‘rad’, ‘ddrad’, ‘pairddrad), and which enzyme cut site overhangs are expected to be present on the reads. Change the following lines in your params files to look like this:
ipsimdata/pairddrad_example_R*.fastq.gz ## [2] [raw_fastq_path]: Location of raw non-demultiplexed fastq files
ipsimdata/pairddrad_example_barcodes.txt ## [3] [barcodes_path]: Location of barcodes file
pairddrad ## [7] [datatype]: Datatype (see docs): rad, gbs, ddrad, etc.
TGCAG, CGG ## [8] [restriction_overhang]: Restriction overhang (cut1,) or (cut1, cut2)
After you change these parameters choose File->Save.
Once we start running the analysis ipyrad will create several new directories to
hold the output of each step for this assembly. By default the new directories
are created in the project_dir directory and use the prefix specified by the
assembly_name parameter. For this example assembly all the intermediate
directories will be of the form: ~/ipyrad-workshop/peddrad_*.
Note: Again, the
~notation indicates a shortcut for the user home directory, in this case/home/jovyan.
Input data format
Before we get started, let’s take a look at what the raw data looks like.
Remember that you can use zcat and head to do this. Since we already looked
at R1 in the last section, lets look at R2 this time:
## zcat: unZip and conCATenate the file to the screen
## head -n 20: Just take the first 20 lines of input
$ zcat ipsimdata/pairddrad_example_R2_.fastq.gz | head -n 20
@lane1_locus0_2G_0_0 2:N:0:
CGGGGTTAAGAGGCCAGTTAACTGCAGCGGGATCGCGCACCATAGCGGCCGTGCCTACGAGTCAGATGTCACTTTTCAGACGCTCATGGAAGTGAGTGCA
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_2G_0_1 2:N:0:
CGGGGTTAAGAGGCCAGTTAACTGCAGCGGGATCGCGCACCATAGCGGCCGTGCCTACGAGTCAGATGTCACTTTTCAGACGCTCATGGAAGTGAGTGCA
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_2G_0_2 2:N:0:
CGGGGTTAAGAGGCCAGGTAACTGCAGCGGGATCGCGCACCATAGCGGCCGTGCCTACGAGTCAGATGTCACTTTTCAGACGCTCATGGAAGTGAGTGCA
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_2G_0_3 2:N:0:
CGGGGTTAAGAGGCCAGTTAACTGCAGCGGGATCGCGCACCATAGCGGCCGTGCCTACGAGTCAGATGTCACTTTTCAGACGCTCATGGAAGTGAGTGCA
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_2G_0_4 2:N:0:
CGGGGTTAAGAGGCCAGTTAACTGCAGCGGGATCGCGCACCATAGCGGCCGTGCCTACGAGTCAGATGTCACTTTTCAGACGCTCATGGAAGTGAGTGCA
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
The simulated data are 100bp paired-end reads generated as ddRAD, meaning there will be two overhang sequences. In this case the ‘common’ cutter leaves the CGG overhang. Can you find this sequence in the raw data? One would think that the second overhang sequence would come at the end of the R2 reads, so what is going on with the orientation of these reads?
Step 1: Demultiplexing the raw data
Since the raw data is still just a huge pile of reads, we need to split it up
and assign each read to the sample it came from. This will create a new
directory called peddrad_fastqs with one .gz file per sample.
Note on step 1: Sometimes, rather than returning the raw data, sequencing facilities will give the data pre-demultiplexed to samples. This situation only slightly modifies step 1, and does not modify further steps, so we will refer you to the full ipyrad tutorial for guidance in this case.
Now lets run step 1! For the simulated data this will take just a few moments.
Special Note: It’s good practice to specify the number of cores with the
-cflag. If you do not specify the number of cores ipyrad assumes you want all of them. Binder instances run on 1 core, so we will specify-c 1for all ipyrad assembly steps.
## -p the params file we wish to use
## -s the step to run
## -c run on 1 core
$ ipyrad -p params-peddrad.txt -s 1 -c 1
-------------------------------------------------------------
ipyrad [v.0.9.59]
Interactive assembly and analysis of RAD-seq data
-------------------------------------------------------------
Parallel connection | jupyter-dereneaton-2dipyrad-2d975c3axu: 1 cores
Step 1: Demultiplexing fastq data to Samples
[####################] 100% 0:00:09 | sorting reads
[####################] 100% 0:00:05 | writing/compressing
Parallel connection closed.
In-depth operations of running an ipyrad step
Any time ipyrad is invoked it performs a few housekeeping operations:
- Load the assembly object - Since this is our first time running any steps we need to initialize our assembly.
- Start the parallel cluster - ipyrad uses a parallelization library called ipyparallel. Every time we start a step we fire up the parallel clients. This makes your assemblies go smokin’ fast.
- Do the work - Actually perform the work of the requested step(s) (in this case demultiplexing reads to samples).
- Save, clean up, and exit - Save the state of the assembly, and spin down the ipyparallel cluster.
As a convenience ipyrad internally tracks the state of all your steps in your
current assembly, so at any time you can ask for results by invoking the -r
flag. We also use the -p argument to tell it which params file (i.e., which
assembly) we want it to print stats for.
## -r fetches informative results from currently executed steps
$ ipyrad -p params-peddrad.txt -r
loading Assembly: peddrad
from saved path: ~/ipyrad-workshop/peddrad.json
Summary stats of Assembly peddrad
------------------------------------------------
state reads_raw
1A_0 1 19835
1B_0 1 20071
1C_0 1 19969
1D_0 1 20082
2E_0 1 20004
2F_0 1 19899
2G_0 1 19928
2H_0 1 20110
3I_0 1 20078
3J_0 1 19965
3K_0 1 19846
3L_0 1 20025
Full stats files
------------------------------------------------
step 1: ./peddrad_fastqs/s1_demultiplex_stats.txt
step 2: None
step 3: None
step 4: None
step 5: None
step 6: None
step 7: None
If you want to get even more info, ipyrad tracks all kinds of wacky stats and saves them to a file inside the directories it creates for each step. For instance, to see full stats for step 1 (the wackyness of the step 1 stats at this point isn’t very interesting, but we’ll see stats for later steps are more verbose):
$ cat peddrad_fastqs/s1_demultiplex_stats.txt
raw_file total_reads cut_found bar_matched
pairddrad_example_R1_.fastq 239812 239812 239812
pairddrad_example_R2_.fastq 239812 239812 239812
sample_name total_reads
1A_0 19835
1B_0 20071
1C_0 19969
1D_0 20082
2E_0 20004
2F_0 19899
2G_0 19928
2H_0 20110
3I_0 20078
3J_0 19965
3K_0 19846
3L_0 20025
sample_name true_bar obs_bar N_records
1A_0 CATCATCAT CATCATCAT 19835
1B_0 CCAGTGATA CCAGTGATA 20071
1C_0 TGGCCTAGT TGGCCTAGT 19969
1D_0 GGGAAAAAC GGGAAAAAC 20082
2E_0 GTGGATATC GTGGATATC 20004
2F_0 AGAGCCGAG AGAGCCGAG 19899
2G_0 CTCCAATCC CTCCAATCC 19928
2H_0 CTCACTGCA CTCACTGCA 20110
3I_0 GGCGCATAC GGCGCATAC 20078
3J_0 CCTTATGTC CCTTATGTC 19965
3K_0 ACGTGTGTG ACGTGTGTG 19846
3L_0 TTACTAACA TTACTAACA 20025
no_match _ _ 0
Step 2: Filter reads
This step filters reads based on quality scores and maximum number of uncalled bases, and can be used to detect Illumina adapters in your reads, which is sometimes a problem under a couple different library prep scenarios. We know the simulated data is unrealistically clean, so lets just pretend it’s more like the Anolis data we looked at earlier, i.e. some slight adapter contamination, and a little noise toward the 3’ end of the reads. To account for this we will trim reads to 75bp and set adapter filtering to be quite aggressive.
Note: Here, we are just trimming the reads for the sake of demonstration. In reality you’d want to be more careful about choosing these values.
Return to the tab with the params file open in the text editor (or open it again if you closed it and change the following two parameter settings:
2 ## [16] [filter_adapters]: Filter for adapters/primers (1 or 2=stricter)
0, 75, 0, 0 ## [25] [trim_reads]: Trim raw read edges (R1>, <R1, R2>, <R2) (see docs)
$ ipyrad -p params-peddrad.txt -s 2 -c 1
loading Assembly: peddrad
from saved path: ~/ipyrad-workshop/peddrad.json
-------------------------------------------------------------
ipyrad [v.0.9.59]
Interactive assembly and analysis of RAD-seq data
-------------------------------------------------------------
Parallel connection | jupyter-dereneaton-2dipyrad-2d975c3axu: 1 cores
Step 2: Filtering and trimming reads
[####################] 100% 0:00:21 | processing reads
Parallel connection closed.
The filtered files are written to a new directory called peddrad_edits. Again,
you can look at the results from this step and some handy stats tracked
for this assembly.
## View the output of step 2
$ cat peddrad_edits/s2_rawedit_stats.txt
reads_raw trim_adapter_bp_read1 trim_adapter_bp_read2 trim_quality_bp_read1 trim_quality_bp_read2 reads_filtered_by_Ns reads_filtered_by_minlen reads_passed_filter
1A_0 19835 331 379 0 0 0 0 19835
1B_0 20071 347 358 0 0 0 0 20071
1C_0 19969 318 349 0 0 0 0 19969
1D_0 20082 350 400 0 0 0 0 20082
2E_0 20004 283 469 0 0 0 0 20004
2F_0 19899 306 442 0 0 0 0 19899
2G_0 19928 302 424 0 0 0 0 19928
2H_0 20110 333 462 0 0 0 0 20110
3I_0 20078 323 381 0 0 0 0 20078
3J_0 19965 310 374 0 0 0 0 19965
3K_0 19846 277 398 0 0 0 0 19846
3L_0 20025 342 366 0 0 0 0 20025
## Get current stats including # raw reads and # reads after filtering.
$ ipyrad -p params-peddrad.txt -r
You might also take a closer look at the filtered reads:
$ zcat peddrad_edits/1A_0.trimmed_R1_.fastq.gz | head -n 12
@lane1_locus0_1A_0_0 1:N:0:
TGCAGTTTAACTGTTCAAGTTGGCAAGATCAAGTCGTCCCTAGCCCCCGCGTCCGTTTTTACCTGGTCGCGGTCC
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_1A_0_1 1:N:0:
TGCAGTTTAACTGTTCAAGTTGGCAAGATCAAGTCGTCCCTAGCCCCCGCGTCCGTTTTTACCTGGTCGCGGTCC
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
@lane1_locus0_1A_0_2 1:N:0:
TGCAGTTTAACTGTTCAAGTTGGCAAGATCAAGTCGTCCCTAGCCCCCGCGTCCGTTTTTACCTGGTCGCGGTCC
+
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
This is actually really cool, because we can already see the results of our applied parameters. All reads have been trimmed to 75bp.
Step 3: denovo clustering within-samples
For a de novo assembly, step 3 de-replicates and then clusters reads within
each sample by the set clustering threshold and then writes the clusters to new
files in a directory called peddrad_clust_0.85. Intuitively, we are trying to
identify all the reads that map to the same locus within each sample. You can see the default value is 0.85, so our default directory is named accordingly. This value dictates the percentage of sequence similarity that
reads must have in order to be considered reads at the same locus.
NB: The true name of this output directory will be dictated by the value you set for the
clust_thresholdparameter in the params file.
You’ll more than likely want to experiment with this value, but 0.85 is a reliable default, balancing over-splitting of loci vs over-lumping. Don’t mess with this until you feel comfortable with the overall workflow, and also until you’ve learned about branching assemblies.
NB: What is the best clustering threshold to choose? “It depends.”
It’s also possible to incorporate information from a reference genome to improve clustering at this step, if such a resources is available for your organism (or one that is relatively closely related). We will not cover reference based assemblies in this workshop, but you can refer to the ipyrad documentation for more information.
Note on performance: Steps 3 and 6 generally take considerably longer than any of the steps, due to the resource intensive clustering and alignment phases. These can take on the order of 10-100x as long as the next longest running step. This depends heavily on the number of samples in your dataset, the number of cores, the length(s) of your reads, and the “messiness” of your data.
Now lets run step 3:
$ ipyrad -p params-peddrad.txt -s 3 -c 1
loading Assembly: peddrad
from saved path: ~/ipyrad-workshop/peddrad.json
-------------------------------------------------------------
ipyrad [v.0.9.59]
Interactive assembly and analysis of RAD-seq data
-------------------------------------------------------------
Parallel connection | jupyter-dereneaton-2dipyrad-2d975c3axu: 1 cores
Step 3: Clustering/Mapping reads within samples
[####################] 100% 0:00:11 | concatenating
[####################] 100% 0:00:02 | join merged pairs
[####################] 100% 0:00:03 | join unmerged pairs
[####################] 100% 0:00:01 | dereplicating
[####################] 100% 0:00:12 | clustering/mapping
[####################] 100% 0:00:00 | building clusters
[####################] 100% 0:00:00 | chunking clusters
[####################] 100% 0:04:00 | aligning clusters
[####################] 100% 0:00:00 | concat clusters
[####################] 100% 0:00:00 | calc cluster stats
Parallel connection closed.
In-depth operations of step 3:
- concatenating - If multiple fastq edits per sample then pile them all together
- join merged/unmerged pairs - For pared-end data merge overlapping reads per mate pair
- dereplicating - Merge all identical reads
- clustering - Find reads matching by sequence similarity threshold
- building clusters - Group similar reads into clusters
- chunking clusters - Subsample cluster files to improve performance of alignment step
- aligning clusters - Align all clusters
- concat clusters - Gather chunked clusters into one full file of aligned clusters
- calc cluster stats - Just as it says.
Again we can examine the results. The stats output tells you how many clusters were found (‘clusters_total’), and the number of clusters that pass the mindepth thresholds (‘clusters_hidepth’). We’ll go into more detail about mindepth settings in some of the advanced tutorials.
$ ipyrad -p params-peddrad.txt -r
Summary stats of Assembly peddrad
------------------------------------------------
state reads_raw reads_passed_filter refseq_mapped_reads refseq_unmapped_reads clusters_total clusters_hidepth
1A_0 3 19835 19835 19835 0 1000 1000
1B_0 3 20071 20071 20071 0 1000 1000
1C_0 3 19969 19969 19969 0 1000 1000
1D_0 3 20082 20082 20082 0 1000 1000
2E_0 3 20004 20004 20004 0 1000 1000
2F_0 3 19899 19899 19899 0 1000 1000
2G_0 3 19928 19928 19928 0 1000 1000
2H_0 3 20110 20110 20110 0 1000 1000
3I_0 3 20078 20078 20078 0 1000 1000
3J_0 3 19965 19965 19965 0 1000 1000
3K_0 3 19846 19846 19846 0 1000 1000
3L_0 3 20025 20025 20025 0 1000 1000
Again, the final output of step 3 is dereplicated, clustered files for
each sample in ./peddrad_clust_0.85/. You can get a feel for what
this looks like by examining a portion of one of the files.
$ zcat peddrad_clust_0.85/1A_0.clustS.gz | head -n 18
0121ac19c8acb83e5d426007a2424b65;size=18;*
TGCAGTTGGGATGGCGATGCCGTACATTGGCGCATCCAGCCTCGGTCATTGTCGGAGATCTCACCTTTCAACGGTnnnnTGAATGGTCGCGACCCCCAACCACAATCGGCTTTGCCAAGGCAAGGCTAGAGACGTGCTAAAAAAACTCGCTCCG
521031ed2eeb3fb8f93fd3e8fdf05a5f;size=1;+
TGCAGTTGGGATGGCGATGCCGTACATTGGCGCATCCAGCCTCGGTCATTGTCGGAGATCTCACCTTTCAACGGTnnnnTGAATGGTCGCGACCCCCAACCACAATCGGCTTTGCCAAGGCAAGGCTAGAGAAGTGCTAAAAAAACTCGCTCCG
//
//
014947fbb43ef09f5388bbd6451bdca0;size=12;*
TGCAGGACTGCGAATGACGGTGGCTAGTACTCGAGGAAGGGTCGCACCGCAGTAAGCTAATCTGACCCTCTGGAGnnnnACCAGTGGTGGGTAAACACCTCCGATTAAGTATAACGCTACGTGAAGCTAAACGGCACCTATCACATAGACCCCG
072588460dac78e9da44b08f53680da7;size=8;+
TGCAGGTCTGCGAATGACGGTGGCTAGTACTCGAGGAAGGGTCGCACCGCAGTAAGCTAATCTGACCCTCTGGAGnnnnACCAGTGGTGGGTAAACACCTCCGATTAAGTATAACGCTACGTGAAGCTAAACGGCACCTATCACATAGACCCCG
fce2e729af9ea5468bafbef742761a4b;size=1;+
TGCAGGACTGCGAATGACGGTGGCTAGTACTCGAGGAAGGGTCGCACCGCAGCAAGCTAATCTGACCCTCTGGAGnnnnACCAGTGGTGGGTAAACACCTCCGATTAAGTATAACGCTACGTGAAGCTAAACGGCACCTATCACATAGACCCCG
24d23e93688f17ab0252fe21f21ce3a7;size=1;+
TGCAGGTCTGCGAATGACGGTGGCTAGTACTCGAGGAAGGGTCGCACCGCAGAAAGCTAATCTGACCCTCTGGAGnnnnACCAGTGGTGGGTAAACACCTCCGATTAAGTATAACGCTACGTGAAGCTAAACGGCACCTATCACATAGACCCCG
ef2c0a897eb5976c40f042a9c3f3a8ba;size=1;+
TGCAGGTCTGCGAATGACGGTGGCTAGTACTCGAGGAAGGGTCGCACCGCAGTAAGCTAATCTGACCCTCTGGAGnnnnACCAGTGGTGGGTAAACACCTCCGATTAAGTATAACGCTACGTGAAGCTAAACGGCACCTATCACATCGACCCCG
//
//
Reads that are sufficiently similar (based on the above sequence similarity threshold) are grouped together in clusters separated by “//”. The first cluster above is probably homozygous with some sequencing error. The second cluster is probably heterozygous with some sequencing error. We don’t want to go through and ‘decide’ by ourselves for each cluster, so thankfully, untangling this mess is what steps 4 & 5 are all about. ipyrad CLI Part II (steps 4-7) is here.
However, this is probably a good time for a coffee break.