The ipyrad.analysis module: PCA
As part of the ipyrad.analysis toolkit we’ve created convenience functions for
easily performing exploratory principal component analysis (PCA) on your data.
PCA is a very standard dimension-reduction technique that is often used to get
a general sense of how samples are related to one another. PCA has the advantage
over STRUCTURE type analyses in that it is very fast. Similar to STRUCTURE, PCA
can be used to produce simple and intuitive plots that can be used to guide
downstream analysis. These are three very nice papers that talk about the
application and interpretation of PCA in the context of population genetics:
- Reich et al (2008) Principal component analysis of genetic data
- Novembre & Stephens (2008) Interpreting principal component analyses of spatial population genetic variation
- McVean (2009) A genealogical interpretation of principal components analysis
A note on Jupyter/IPython
Jupyter notebooks are primarily a way to generate reproducible scientific analysis workflows in python. ipyrad analysis tools are best run inside Jupyter notebooks, as the analysis can be monitored and tweaked and provides a self-documenting workflow.
The rest of the materials in this part of the workshop assume you are running all code in cells of a jupyter notebook.
PCA analyses
Create a new notebook for the PCA
Similar how you’ve previously opened the Terminal, now open a new “Python 3” notebook by clicking on New.
The empirical dataset (Cheetah)
For this exercise we’ll use empirical data, so it’ll be more fun and also a bit more like running a PCA in real life than doing so with the simulated data would be. This data is accessible here:
/data/subset_cheetah.snps.hdf5
This .hdf5 file is one of the results files writen to the outputs directory of ipyrad after step 7.
Import ipyrad.analysis module
For this analysis, we’ll use python. The import keyword directs python to load a module into the currently running context. This is very similar to the library() function in R. We begin by importing the ipyrad analysis module. Copy the code below into a notebook cell and click run.
import ipyrad.analysis as ipa
The
as ipapart here creates a short synonym so that we can refer toipyrad.analysisasipa, which is just faster to type.
Quick guide (tl;dr)
The following cell shows the quickest way to results using the small simulated dataset. Complete explanation of all of the features and options of the PCA module is the focus of the rest of this tutorial. Copy this code into a new notebook cell (you can add new cells with the + button on the toolbar) and run it.
data = "/data/subset_cheetah.snps.hdf5"
## Create the pca object
pca = ipa.pca(data)
## Run the analysis
pca.run()
## Bam!
pca.draw()
Note: In this block of code, the
#at the beginning of a line indicates to python that this is a comment, so it doesn’t try to run this line. This is a very handy thing if you want to add or remove lines of code from an analysis without deleting them. Simply comment them out with the#!
Full guide
Simple PCA from ipyrad hdf5 file
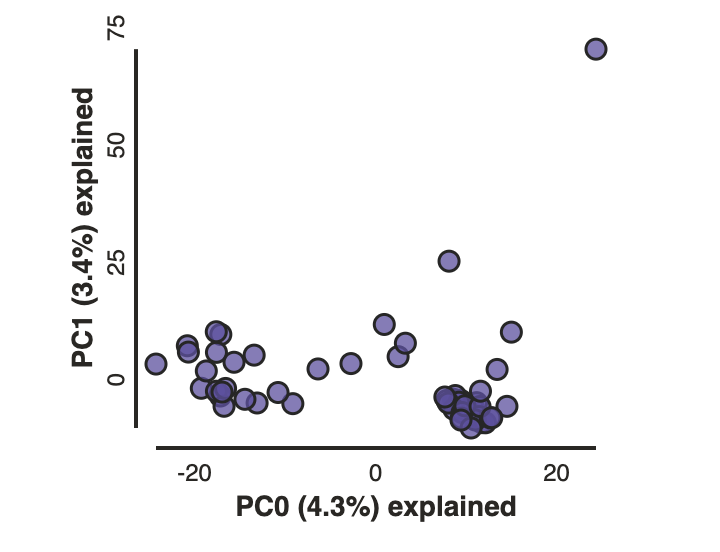
In the most common use, you’ll want to plot the first two PCs, then inspect the output, remove any obvious outliers, and then redo the PCA.
## Path to the input data in snps.hdf5 format
data = "/data/subset_cheetah.snps.hdf5"
pca = ipa.pca(data)
Note: Here we use the hdf5 database file with SNPs generated with ipyrad from the cheetah data. The database file contains the genotype calls information as well as linkage information that is used for subsampling unlinked SNPs and bootstrap resampling.
Note: The
ipyrad.analysis.pcamodule can also read in data from any vcf file, so it’s possible to quickly generate PCA plots for any vcf from any dataset.
Now construct the default plot, which shows all samples and PCs 1 and 2.
pca.run()
pca.draw()

Population assignment for sample colors
Typically it is useful to color points in a PCA by some a priori grouping, such
as presumed population, or by experimental treatment groups, etc. To facilitate
this it is possible to specify population assignments in a dictionary. The
format of the dictionary should have populations as keys and lists of samples
as values. Sample names need to be identical to the names in the input dataset,
which we can verify with the names property of the PCA object. Open a new cell
and type this:
pca.names
This will show you a list of sample names.
Here we create a python ‘dictionary’, which is a key/value pair data structure. The keys are the population names, in our case cheetah subspecies, and the values are the lists of samples that belong to those populations. You can copy and paste this into a new cell in your notebook.
imap = {"A.j.jubatus":['SRR19760918','SRR19760920','SRR19760921','SRR19760924','SRR19760927','SRR19760928','SRR19760942','SRR19760946','SRR19760947','SRR19760956'],
"A.j.soemmeringii":['SRR19760910','SRR19760912','SRR19760954','SRR19760955','SRR19760957','SRR19760958','SRR19760959','SRR19760960','SRR19760961','SRR19760962'],
"A.j.venaticus":['SRR19760950','SRR19760951','SRR19760953'],
"Outgroup":['SRR19760949']}
Now create the pca object with the input data again, this time passing
in the new dictionary as the second argument and specifying this as the imap,
and plot the new figure. We can also easily add a title to our PCA plots
with the label= argument.
pca = ipa.pca(data, imap=imap)
pca.run()
pca.draw(label="Sims colored by pop")
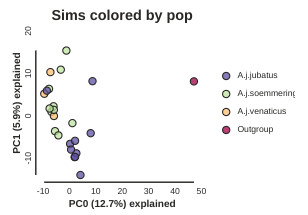
This is just much nicer looking now, and it’s also much more straightforward to interpret.
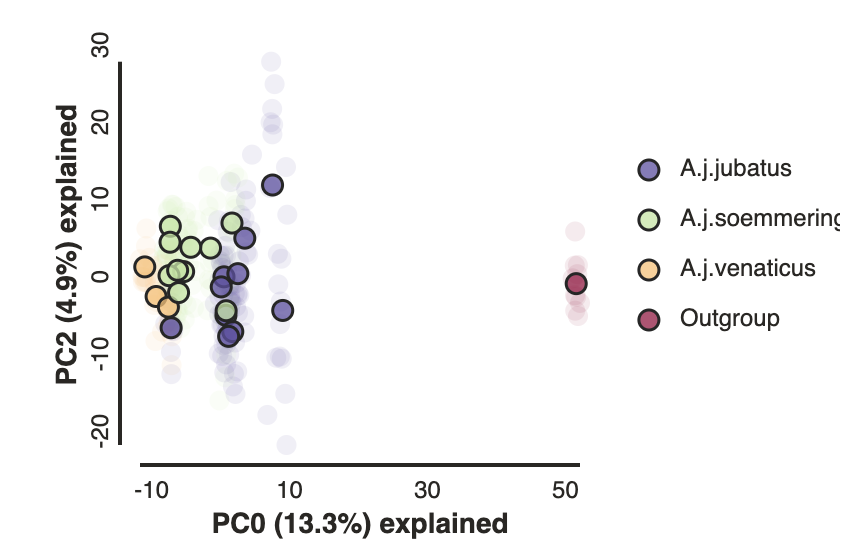
Removing “bad” samples and replotting.
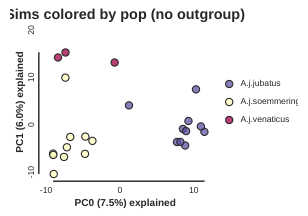
In PC analysis, it’s common for “bad” samples to dominate several of the first PCs, and thus “pop out” in a degenerate looking way. Bad samples of this kind can often be attributed to poor sequence quality or sample misidentifcation. Samples with lots of missing data tend to pop way out on their own, causing distortion in the signal in the PCs. Normally it’s best to evaluate the quality of the sample, and if it can be seen to be of poor quality, to remove it and replot the PCA. In our dataset, we don’t really have bad samples, but we do have an outlier: the outgroup (which in this case is Puma concolor). Because we’re intersted in cheetah only, let’s remove the outgroup sample and rerun the PCA.
Note: We make a lot of use of the interactivity of jupyter notebooks in the ipyrad.analysis tools. In the PCA you can ‘hover’ over points to reveal their sample ID.
The easiest way to achieve this is to simply remove the sample from the imap
file and run the PCA again.
imap = {"A.j.jubatus":['SRR19760918','SRR19760920','SRR19760921','SRR19760924','SRR19760927','SRR19760928','SRR19760942','SRR19760946','SRR19760947','SRR19760956'],
"A.j.soemmeringii":['SRR19760910','SRR19760912','SRR19760954','SRR19760955','SRR19760957','SRR19760958','SRR19760959','SRR19760960','SRR19760961','SRR19760962'],
"A.j.venaticus":['SRR19760950','SRR19760951','SRR19760953']}
pca = ipa.pca(data, imap=imap)
pca.run()
pca.draw(label="Sims colored by pop (no outgroup)")
Subsampling with replication
By default run() will randomly subsample one SNP per RAD locus to reduce the
effect of linkage on your results. This can be turned off by setting
subsample=False. However, subsampling unlinked SNPs is generally a good
idea for PCA analyses since you want to remove the effects of linkage from your
data. It also presents a convenient way to explore the confidence in your
results. By using the option nreplicates you can run many replicate analyses
that subsample a different random set of unlinked SNPs each time. The replicate
results are drawn with a lower opacity and the centroid of all the points for
each sample is plotted as a black point.
pca.run(nreplicates=10)
pca.draw();
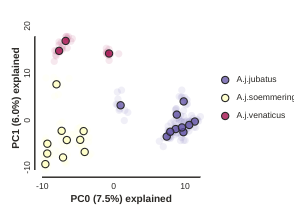
Plotting PCs other than 0 and 1
Even though PC 0 and 1 by definition explain the most variance in the data,
it is still often useful to examine other PCs. You can do this by specifying
which PCs to plot in in the call to draw.
pca.draw(0,2)
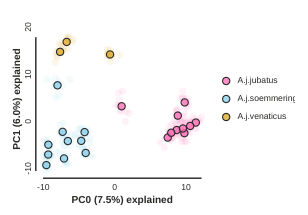
Custom color points
Another nice feature of the draw method is the ability to pass in any custom
color that you like for each population. You can do this in a couple different
ways, but the most straightforward is just to pass in a list of valid color names
from the ‘named colors’ matplotlib documentation.
pca.draw(colors=["hotpink", "skyblue", "goldenrod"])
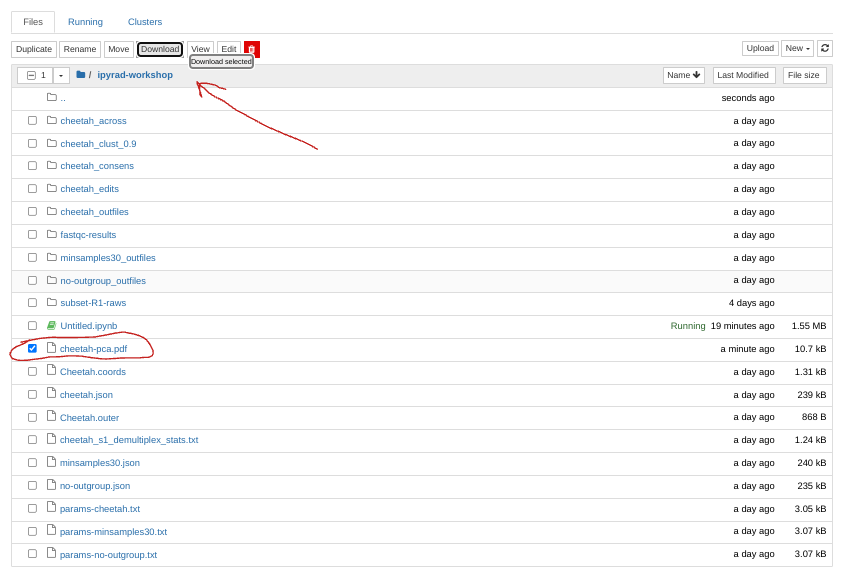
Saving a PCA plot to a file
You can save the figure as a PDF or SVG automatically by passing an outfile
argument to the .draw() function.
# The outfile must end in either `.pdf` or `.svg`
pca.draw(outfile="cheetah-pca.pdf")
This will save cheetah-pca.pdf into your notebook environment. You can open the
jupyter file browser and open or download the pdf from there.
Dealing with missing data in PCA
PCA can be extremely sensitive to missing data if there is any pattern at all in the missingness.
We offer three algorithms for imputing missing data:
sample: Randomly sample genotypes based on the frequency of alleles within (user-defined) populations (imap).kmeans: Randomly sample genotypes based on the frequency of alleles in (kmeans cluster-generated) populations.- ‘random’: This is less an imputation method than an attempt to reduce the bias of assigning all missing data to the ancestral state. Instead missing data are randomly assigned to ancestral or derived states.
None(default): All missing values are imputed with zeros (ancestral allele).
Here is an example of how to select an impute_method:
pca1 = ipa.pca(
data=data,
imap=imap,
impute_method='sample',
)
A fourth approach is to simply enforce an lower bound on missing data with
the mincov parameter. mincov specifies the minimum coverage threshold
below which a snp is excluded from the analysis.
This code will exclude any snp not present in 100% of samples, a very strict threshold.
pca2 = ipa.pca(
data=data,
mincov=1.0,
impute_method=None,
)
We encourage you to experiment with different imputation schemes and missing data thresholds when analysing your own data later.
More to explore
The ipyrad.analysis.pca module has many more features that we just don’t have
time to go over, but you might be interested in checking them out later:
Excercise: Plot some PCAs for different gecko species
There are several vcf files for different Galapagos gecko species (and a few
random other ones) in the /data/vcfs directory. See if you can plot some PCAs
for some of these different species. Can you see one, two or more possible
populations in each of these species?